Systematic literature reviews (SLRs) are essential for gathering comprehensive evidence to support decision-making. While PubMed, Cochrane, and Embase are well-known resources, there are lesser-known databases that offer valuable insights and data. Exploring these unconventional databases can uncover hidden gems that significantly enhance the robustness and breadth of your SLRs. Figure 1 summarises seven such underutilised resources.
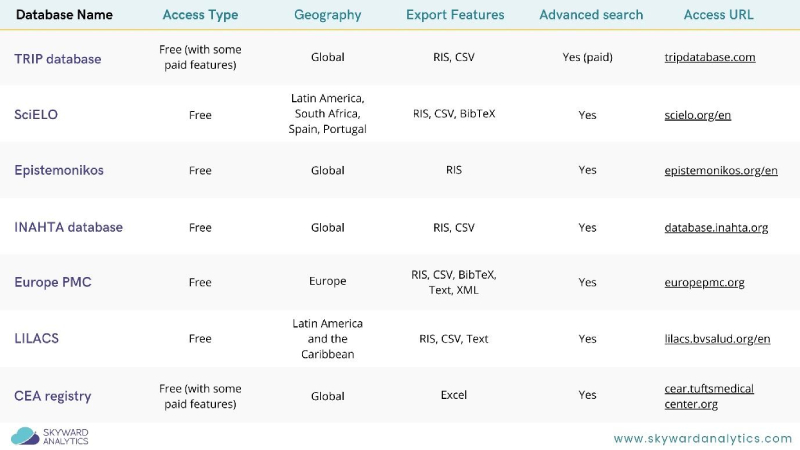
1. TRIP Database (Turning Research Into Practice )
The TRIP Database is a clinical search engine designed to allow health professionals to rapidly identify high-quality clinical evidence. It indexes evidence-based content to support clinical practice and decision-making, ensuring your SLRs are based on the highest quality evidence available.
2. SciELO (Scientific Electronic Library Online)
SciELO provides open-access to scientific literature from Latin America, Spain, Portugal, and South Africa. It offers a treasure trove of articles in public health, social sciences, and health sciences, making it an excellent resource for regional studies and diverse perspectives.
3. Epistemonikos
A comprehensive database of health evidence, Epistemonikos aggregates SLRs and other types of evidence from multiple sources. Its user-friendly interface and focus on health make it a powerful tool for uncovering high-quality evidence across a range of medical and health-related topics.
4. International HTA Database
Maintained by the International Network of Agencies for Health Technology Assessment (INAHTA), this database provides reports from HTA agencies worldwide. It could be essential for finding grey literature and HTA reports that might not be indexed elsewhere.
5. Europe PMC (European PubMed Central)
Europe PMC offers access to a broad range of biomedical literature, including research articles, reviews, and patents. It integrates literature from PubMed as well as additional sources, providing a more extensive search for European studies and beyond.
6. LILACS (Latin-American scientific literature in health science)
LILACS, part of the Virtual Health Library (VHL), is the most important and comprehensive index of scientific and technical literature in Latin America and the Caribbean. It includes various types of documents, offering valuable insights into regional health issues.
7. CEA Registry (Cost-Effectiveness Analysis registry)
The CEA Registry, managed by Tufts Medical Centre, is a comprehensive database of cost-effectiveness analyses in health and medicine. It provides detailed information on the economic evaluations of health interventions, crucial for HEOR studies focusing on cost-effectiveness.
CONCLUDING REMARKS
Venturing beyond conventional databases can significantly enhance the depth and quality of your SLRs. Expanding your SLR toolkit with these free databases not only enhances the depth of your research but also ensures a broader spectrum of evidence is considered, making your findings more robust and reflective of the global research landscape.
References
- Stevens GA, Fitterling L, Kelly FV. Trip database: turning research into practice for evidence-based care. Medical Reference Services Quarterly. 2017 Oct 2;36(4):391-8.
- Rocha EM, Osaki TH, Kara N, Alves M, Moral C. SciELO 25 years: The Scientific Electronic Library Online celebrates its 25th anniversary. Arquivos Brasileiros de Oftalmologia. 2023 Dec 11;87(1):e2024-1007.
- Rada G, Perez D, Araya-Quintanilla F, Avila C, Bravo-Soto G, Bravo-Jeria R, Canepa A, Capurro D, Castro-Gutierrez V, Contreras V, Edwards J. Epistemonikos: a comprehensive database of systematic reviews for health decision-making. BMC medical research methodology. 2020 Dec;20:1-7.
- Bellemare CA, Dagenais P, Suzanne K, Béland JP, Bernier L, Daniel CÉ, Gagnon H, Legault GA, Parent M, Patenaude J. Ethics in health technology assessment: a systematic review. International Journal of Technology Assessment in Health Care. 2018 Jan;34(5):447-57.
- Europe PMC Consortium. Europe PMC: a full-text literature database for the life sciences and platform for innovation. Nucleic acids research. 2015 Jan 28;43(D1):D1042-8.
- Manriquez JJ. Searching the LILACS database could improve systematic reviews in dermatology. Archives of dermatology. 2009 Aug 1;145(8):947-68.
- Neumann PJ, Thorat T, Shi J, Saret CJ, Cohen JT. The changing face of the cost-utility literature, 1990–2012. Value in health. 2015 Mar 1;18(2):271-7.










