The integration of artificial intelligence (AI) into Health Technology Assessment (HTA) has become a focal point in the evolving landscape of evidence generation. The National Institute for Health and Care Excellence (NICE) has published a detailed position statement on the integration of AI in evidence generation for HTA. This guidance arrives at a pivotal time when AI’s potential to enhance evidence generation is being keenly explored across healthcare sectors. Below, we delve into the essential facets of NICE’s position and what it means for the future of HTA. Figure 1 summarizes the key points of NICE’s position on AI integration in HTA.
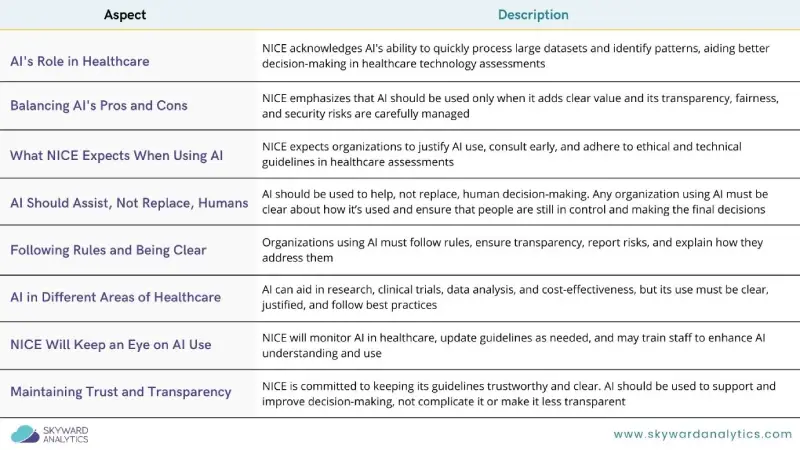
1. Balancing Innovation with Risk
AI’s potential to transform evidence generation in HTA is undeniable. From automating literature reviews to enhancing the design of clinical trials, AI can significantly streamline processes. However, NICE emphasizes the importance of balancing these innovations against inherent risks, such as algorithmic bias, cybersecurity vulnerabilities, and transparency challenges. NICE suggests that AI should only be employed when it clearly adds value, ensuring that the benefits outweigh the potential downsides.
2. Human Oversight
AI should augment human decision-making, not replace it. NICE stresses the importance of keeping humans in the loop, ensuring that AI tools are used to enhance, rather than supplant, human expertise. This approach is crucial for maintaining ethical standards and ensuring the reliability of the evidence produced.
3. Ensuring Transparency and Accountability
NICE insists that organizations using AI in evidence generation must prioritize transparency. This means clearly justifying the use of AI, documenting methodologies, and ensuring that AI-driven results are understandable and accessible to all stakeholders. Transparency is vital to maintaining trust in the AI-generated evidence.
4. Early Engagement with NICE
Organizations considering the use of AI in their evidence generation processes are encouraged to engage with NICE early in the development process. This proactive approach can help ensure that AI methodologies are aligned with NICE’s expectations and regulatory standards from the outset, minimizing potential issues later on.
5. Building Trust through Compliance and Ethical Alignment
Any AI methods used must align with existing UK Government frameworks and ethical guidelines. This includes adherence to data protection laws and ensuring that AI applications are both scientifically and technically sound. Organizations are responsible for ensuring that all AI tools and methodologies meet these stringent standards.
6. Ongoing Monitoring and Adaptation
Given the rapidly evolving nature of AI technology, NICE plans to continually review and update its guidelines to reflect new developments and evidence. This commitment ensures that AI’s integration into HTA remains both innovative and responsible, ultimately supporting improved healthcare outcomes.
CONCLUDING REMARKS
NICE’s position on AI in HTA emphasizes the need for responsible, transparent, and ethical integration of AI technologies. While AI holds significant potential to transform HTA, NICE advocates for a cautious approach that safeguards the integrity of evidence generation and decision-making. By adhering to these guidelines, organizations can harness the power of AI to support and enhance evidence generation, ensuring that AI’s benefits are fully realized without compromising the quality or integrity of healthcare decisions.
References
- National Institute for Health and Care Excellence. (2024). Use of AI in evidence generation: NICE position statement. Retrieved from https://www.nice.org.uk/about/what-we-do/our-research-work/use-of-ai-in-evidence-generation–nice-position-statement











